HCOOCH CH2 H2O
Understanding the Molecular Composition: HCOOCH, CH2, and H2O
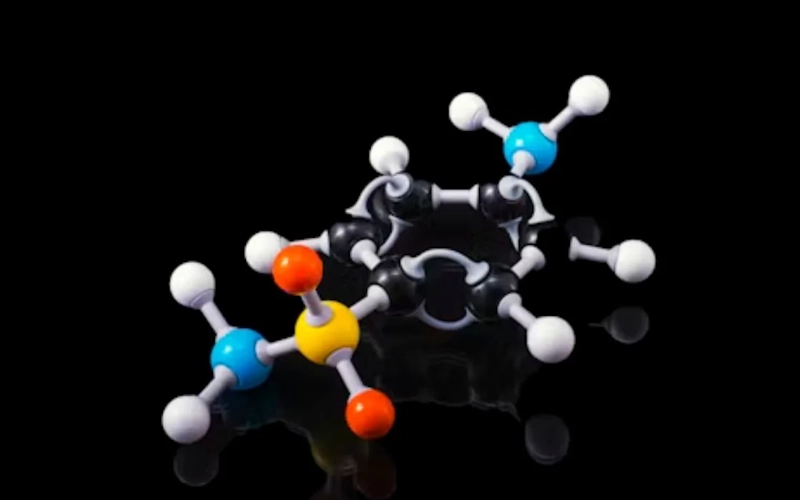
The molecular formula HCOOCH CH2 H2O represents a blend of essential chemical components, combining a formate ester group (HCOOCH), a methylene group (CH2), and water (H2O).
- Formate Ester (HCOOCH): This ester is produced by the reaction between formic acid (HCOOH) and an alcohol, typically methanol. Formate esters, such as HCOOCH CH2 H2O, are important intermediates in organic chemistry, particularly in esterification reactions.
- Methylene Group (CH2): This group connects the ester portion of the molecule to other components. It adds reactivity to the compound and plays a significant role in various chemical reactions that influence the behavior of the compound.
- Water (H2O): Water serves as a critical participant in numerous chemical reactions, including hydrolysis, where it breaks down ester bonds. In the case of HCOOCH CH2 H2O, water supports the stability and solubility of the ester group in diverse solvents.
These components together form a flexible molecular structure, impacting both the physical and chemical properties of the compound. The formate ester group is particularly significant for its role in organic synthesis, while the methylene group introduces additional reactivity. Water stabilizes the structure and aids in transformations.
The Chemistry Behind HCOOCH CH2 H2O
The Esterification Process: Formation of HCOOCH
The formation of formate esters like HCOOCH CH2 H2O begins with an esterification reaction. Formic acid (HCOOH) reacts with methanol (CH3OH) under acidic conditions to yield methyl formate (HCOOCH3). By adding further chemical groups like methylene (CH2) and water (H2O), the formate ester can be modified for particular uses.
Esterification is a cornerstone reaction in organic chemistry, contributing to the creation of a wide range of important compounds. By adjusting reaction conditions, chemists can customize the ester’s properties, such as reactivity, solubility, and stability.
Water’s Role in Hydrolysis Reactions
Water is indispensable in hydrolysis reactions, where it breaks chemical bonds. In the case of formate esters, water can split the ester bond, yielding alcohol and formic acid. This ester hydrolysis process is critical for understanding the reactivity of formate esters such as HCOOCH CH2 H2O.
Hydrolysis allows esterification to be reversed, enabling chemists to regenerate the initial reactants. This feature is especially valuable in processes like chemical recycling and the synthesis of other organic molecules.
The Function of the Methylene Group in the Molecular Framework
The CH2 group in HCOOCH CH2 H2O serves as a bridge between the ester portion and other elements within the molecule. Its role is to enhance the compound’s reactivity, enabling it to engage in various reactions, including esterification and catalytic processes.
The methylene group adds versatility to the molecule by providing reactive sites for further chemical modifications, contributing to the compound’s overall functionality and reactivity.
Properties of HCOOCH CH2 H2O
Physical Characteristics: Solubility, Appearance, and Stability
HCOOCH CH2 H2O typically appears as a clear, colorless liquid with excellent solubility in water and other polar solvents. Its polar nature allows it to dissolve easily in aqueous solutions and other solvents commonly used in organic chemistry.
The compound is generally stable under normal conditions. However, exposure to extreme temperatures or harsh chemicals could lead to its decomposition or transformation. This stability makes HCOOCH CH2 H2O a reliable participant in various chemical reactions and industrial processes.
Chemical Characteristics: Reactivity, Polarity, and Interactions with Other Substances
The reactivity of HCOOCH CH2 H2O is heavily influenced by its ester and methylene groups. These groups make the compound highly reactive in several organic chemistry reactions, including esterification and catalytic reactions. Additionally, its polarity enables it to interact efficiently with polar solvents and reagents, which makes it valuable for various chemical synthesis tasks.
One of the compound’s distinctive chemical properties is its ability to undergo hydrolysis. Water breaks the ester bond, enabling HCOOCH CH2 H2O to be reactive in situations where ester bonds need to be cleaved and re-formed.
Synthesis of HCOOCH CH2 H2O
Steps for Synthesizing Formate Esters
The synthesis of HCOOCH CH2 H2O starts with an esterification reaction between formic acid and methanol. This produces methyl formate, which is then further modified by adding a methylene group (CH2) and water molecules (H2O). Careful control of factors such as temperature, pH, and solvent type is essential for achieving a high yield and purity in the final product.
Once synthesized, the compound can be purified and applied in various fields, ranging from industrial chemistry to environmental science. The simplicity of the synthesis process and the ability to modify its properties make HCOOCH CH2 H2O an attractive compound for both researchers and industrial professionals.
The Role of Acidic Conditions in Ester Formation
Esterification to form HCOOCH CH2 H2O requires acidic conditions to proceed. The acid helps protonate formic acid, making it more reactive. Methanol then attacks the carbonyl carbon, leading to the formation of the ester and the release of water as a byproduct.
Controlling the acidity of the reaction mixture is crucial to ensure the ester is formed correctly. In industrial settings, this process is often expedited with the use of a catalyst to improve the reaction rate and overall efficiency.
Incorporating Methylene Groups and Water into the Synthesis
The methylene group (CH2) is introduced into the formate ester structure through additional chemical reactions, where methylene donors or alkenes provide the necessary components. This step brings added functionality to the compound, making it adaptable for various chemical processes.
Water has a dual role in the synthesis: it serves as both a byproduct of the esterification reaction and a stabilizing agent. It ensures the ester remains in solution, preventing premature decomposition.
Key Facts About HCOOCH CH2 H2O
- Molecular Composition: HCOOCH CH2 H2O is made up of three main components: a formate ester group (HCOOCH), a methylene group (CH2), and water (H2O). This structure is essential for its reactivity and versatility in organic chemistry.
- Formate Ester: The formate ester component (HCOOCH) is formed by the reaction between formic acid (HCOOH) and methanol (CH3OH). It is a significant intermediate in organic synthesis, particularly in esterification reactions.
- Methylene Group: The methylene group (CH2) in HCOOCH CH2 H2O links the ester group with other parts of the molecule, affecting its reactivity. This component allows the molecule to participate in various chemical reactions, adding flexibility to its use.
- Water’s Role: Water (H2O) plays a critical role in the stability and solubility of HCOOCH CH2 H2O. It also participates in hydrolysis reactions, where it breaks down the ester bond, leading to the formation of alcohol and formic acid.
- Reactivity: The compound’s reactivity is influenced by its formate ester and methylene groups. It is involved in esterification, catalytic processes, and hydrolysis, making it important in various chemical transformations.
- Synthesis: HCOOCH CH2 H2O is synthesized through an esterification reaction between formic acid and methanol, with the addition of methylene and water groups. This process requires careful control of reaction conditions such as temperature and acidity.
- Physical Properties: HCOOCH CH2 H2O is typically a colorless, clear liquid with good solubility in water and other polar solvents. Its polar nature makes it useful in organic chemistry, especially in reactions that require solubility in aqueous solutions.
- Chemical Stability: Under standard conditions, the compound remains stable, though extreme temperatures or harsh chemicals may cause decomposition or transformation. It is stable enough for use in industrial and research applications.
- Hydrolysis: HCOOCH CH2 H2O can undergo hydrolysis, where water molecules break down the ester bond. This process is crucial for both reversing esterification and for the recycling of chemical compounds.
- Applications: HCOOCH CH2 H2O has diverse applications in industries such as pharmaceuticals, environmental science, organic synthesis, and as a fuel additive. It plays a role in the synthesis of complex molecules and the development of new drugs.
Conclusion
HCOOCH CH2 H2O is an essential and versatile compound in organic chemistry, with significant applications across pharmaceuticals, environmental science, and organic synthesis. Its distinctive structure, which combines a formate ester, methylene group, and water, gives it reactive properties crucial for various chemical processes. As research into its uses continues, especially in sustainable applications, HCOOCH CH2 H2O’s future potential looks bright. A deeper understanding of its chemistry and properties will open up new opportunities for both industrial and research applications.
Frequently Asked Questions
- What is HCOOCH CH2 H2O?
HCOOCH CH2 H2O is a formate ester compound made from formic acid, methanol, and water. It plays a key role in organic synthesis, esterification reactions, and has applications in various industries. - How is HCOOCH CH2 H2O synthesized?
It is synthesized through an esterification reaction between formic acid and methanol, followed by the integration of methylene groups and water under controlled conditions. - What are the main applications of HCOOCH CH2 H2O?
This compound is used in organic synthesis, as a fuel additive, in solvent applications, and even in the pharmaceutical industry as an intermediate for drug development. - Is HCOOCH CH2 H2O environmentally friendly?
Yes, the components of HCOOCH CH2 H2O, including formate esters and water, are biodegradable, making the compound eco-friendly when handled responsibly. - How does HCOOCH CH2 H2O interact with water?
Water plays a crucial role in hydrolysis reactions, breaking down the ester bond in HCOOCH CH2 H2O and resulting in the formation of alcohol and formic acid.
Keep an eye for more latest news & updates on The Washington Vibes!